Lemtrada Side Effects Thyroid

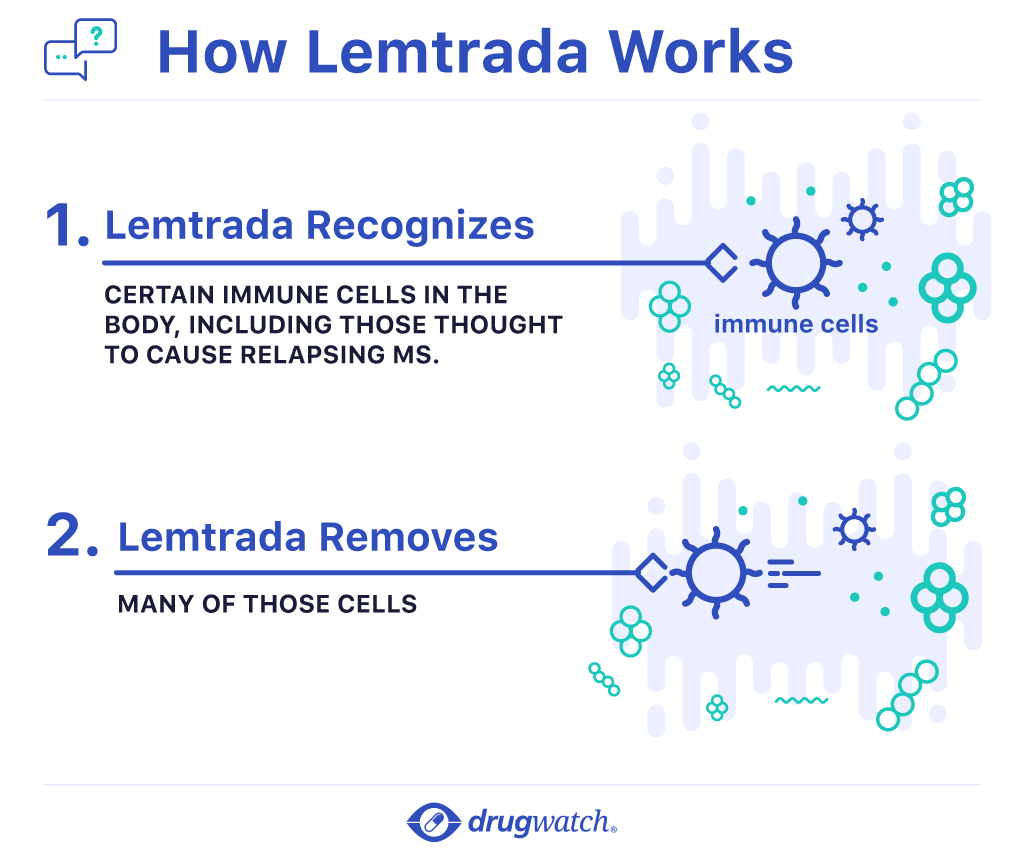
Some people receiving lemtrada develop a condition where the immune cells in your body attack other cells or organs in the body autoimmunity which can be serious and may cause death.
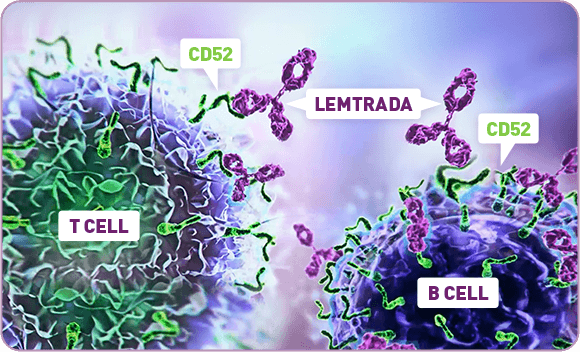
Lemtrada side effects thyroid. Lemtrada is a disease modifying drug dmd for very active relapsing remitting ms. This is not a complete list of side effects and others may occur. See what is the most important information i should know about lemtrada thyroid problems. It reduces the number of relapses by about two thirds 70 compared to taking placebo.
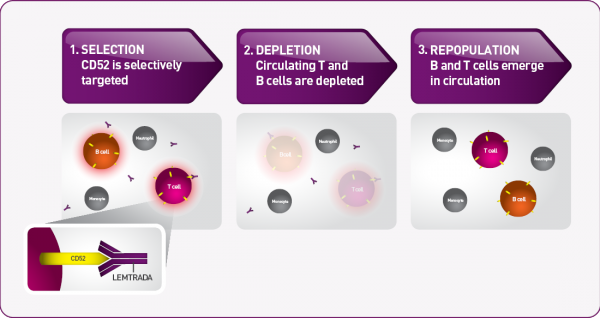
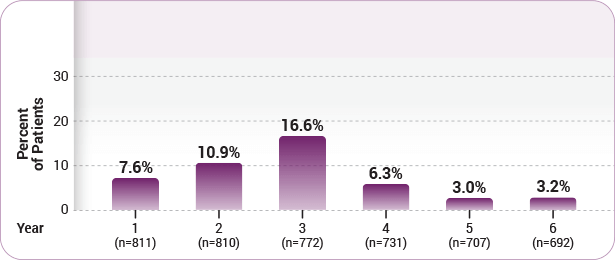
2 according to the package insert thyroid endocrine disorders including autoimmune thyroid disorders have been observed in 36 8 of patients treated with lemtrada 12 mg in the clinical trial. Lemtrada may cause serious side effects including. Common side effects include infusion related reactions which are generally mild and short lived and increased risk of. Lemtrada can cause serious side effects including autoimmune problems infusion reactions stroke tears in your arteries that supply blood to your brain carotid and vertebral arteries some kinds of cancers thyroid problems low blood counts cytopenias inflammation of the liver hemophagocytic lymphohistiocytosis serious infections.
Lemtrada can cause serious side effects including serious autoimmune problems. Serious autoimmune problems may include. What are the possible side effects of lemtrada. A condition with low thyroid hormone levels.
If experienced these tend to have a severe expression. Although not all of these side effects may occur if they do occur they may need medical attention. Call your doctor for medical advice about side effects. Along with its needed effects alemtuzumab the active ingredient contained in lemtrada may cause some unwanted effects.
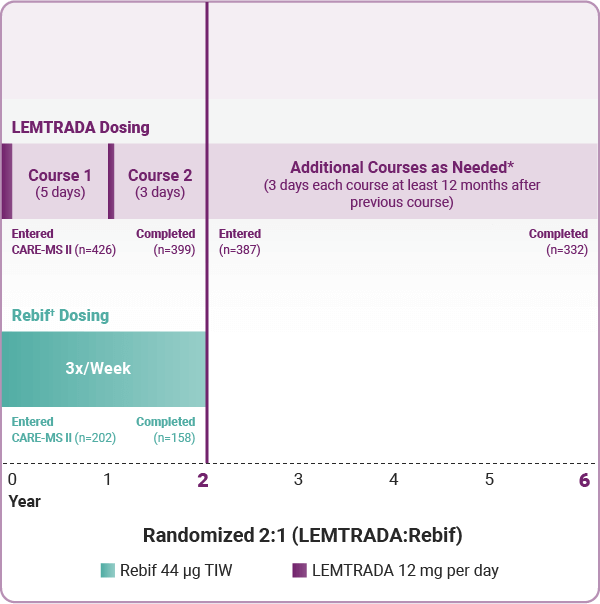
Some people who receive lemtrada may get thyroid problems including an overactive thyroid hyperthyroidism or an underactive thyroid hypothyroidism. You take lemtrada as an intravenous infusion drip in two treatment courses twelve months apart. Side effects requiring immediate medical attention. In november 2018 the fda added its strongest warning to the drug s label about stroke and tears in arteries in the head and neck.
The company s product information indicates different percentages for commonly reported side effects that differ from that reported by the study authors. Lemtrada vial side effects by likelihood and severity common side effects.